library(ceas)
#> Error in get(paste0(generic, ".", class), envir = get_method_env()) :
#> object 'type_sum.accel' not found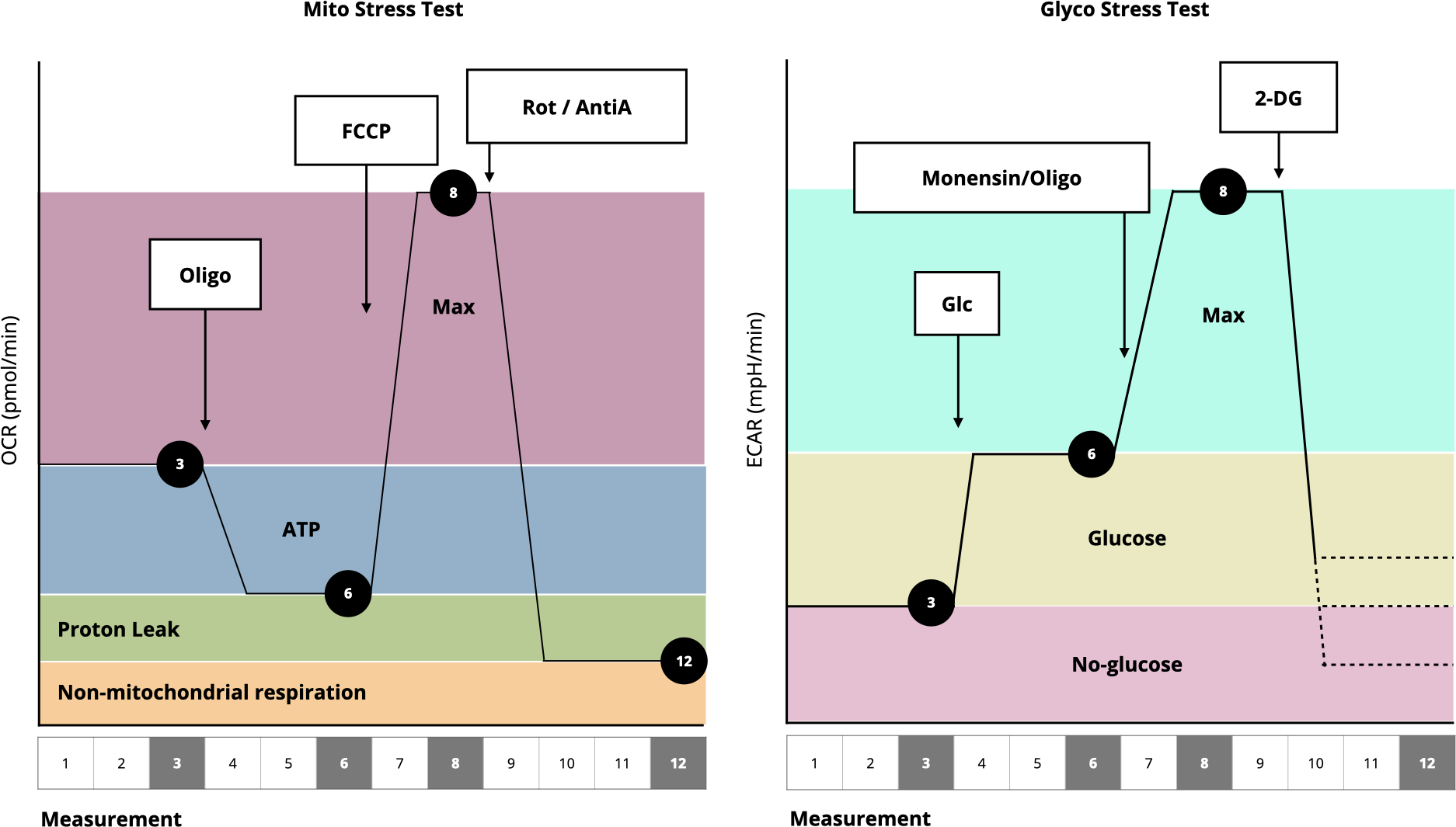
Proton production rate (PPR):
\[\text{PPR} = \frac{\text{ECAR value}}{\text{buffer}}\]
\[\text{PPR}_{\text{mito}} = \frac{10^{\text{pH}-\text{pK}_a}}{1+10^{\text{pH}-\text{pK}_a}} \cdot \frac{\text{H}^+}{\text{O}_2} \cdot \text{OCR}\]
Calculates the proton production from glucose during its conversion to bicarbonate and assuming max \(\frac{\ce{H}^+}{\ce{O2}}\) of 1
\[\text{PPR}_\text{glyc} = \text{PPR} - \text{PPR}_\text{resp}\]
\[ \ce{2H2 + O2 -> 2H2O} \]
Calculates the proton production from glucose during its conversion to \(\ce{lactate + H+}\).
Joules of ATP (JATP) production:
\[ \text{ATP}_{\text{glyc}} = \Bigl(\text{PPR}_\text{glyc} \cdot \frac{\text{ATP}}{\text{lactate}}\Bigl) + \Bigl(\text{MITO}_\text{resp} \cdot 2 \cdot \frac{\text{P}}{\text{O}_\text{glyc}}\Bigl) \]
\[ \frac{\text{ATP}}{\text{lactate}} = 1 \]
with \(\frac{\text{P}}{{\text{O}_\text{glyc}}}\) = 0.167 for glucose (0.242 for glycogen).
\[ \text{ATP}_\text{resp} = \Bigl(\text{coupled MITO}_\text{resp} \cdot 2 \cdot \frac{\text{P}}{\text{O}_\text{oxphos}}\Bigl) + \Bigl(\text{MITO}_\text{resp} \cdot 2 \cdot \frac{\text{P}}{\text{O}_\text{TCA}}\Bigl) \]
with \(\frac{\text{P}}{{\text{O}_\text{oxphos}}}\) = 2.486 and \(\frac{\text{P}}{{\text{O}_\text{TCA}}}\) = 0.167.